Pursuing Excellence in Clinical Supply Management ‒ Topic 4: Business Intelligence
This article describes the current state and challenges to gaining business intelligence insights in the planning and management of the clinical trial supply chain. Moreover, impactful use cases for the use of data analytics in clinical supplies will be exposed. The final section of this article shares thoughts on the path to building and maturing clinical supplies business intelligence excellence.

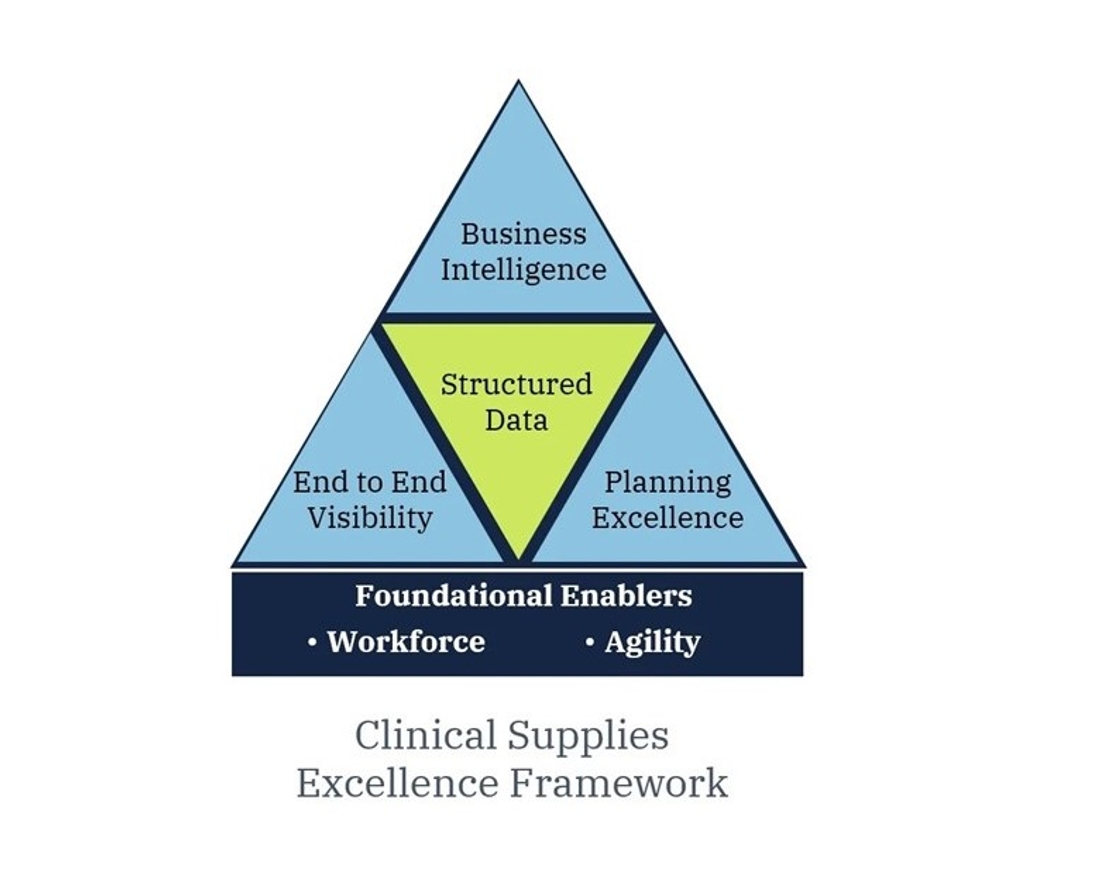
Tenthpin has launched a short series of articles covering aspects of differentiation and excellence for clinical supplies management. These articles are collections of experiences and insights that have come from working with the industry on various initiatives over many years. Previous articles in this series covered excellence in Workforce and Agility, End to End Visibility, and most recently Planning Excellence.
What follows is Article 4: Business Intelligence, the last article of the series.
Introduction
There have been countless articles and examples of how the use of data, typically on a massive scale, and powerful analytics can inform smarter, faster, and ultimately more successful drug development. This touches on many aspects of drug discovery and drug development, as seen in study design optimisation through to study results analysis.
What is the current state of use for business intelligence in the area of clinical supply management?
The story is in the building stage. Many companies are well underway in their usage, and for them the impact is promising with innovative use cases emerging while others, perhaps the majority, remain on the sidelines.
What is preventing a more systematic and innovative use of data to drive awareness of study supply status and support key decisions? The foundation of a rich data set is one major inhibitor, but even for companies that have solved the data challenge, the activity is often not at the scale of effort and success that would match the business impact potential.
CURRENT STATE OF BUSINESS INTELLIGENCE IN CLINICAL SUPPLIES
As mentioned, the key challenge of enabling data analytics capabilities for the clinical supply chain is accessing current state clinical supply chain relevant data and assembling the data into a common structure and location. Without the data being consolidated, structured and combined with historical data, opportunities for reliable and impactful data analytics are limited.
Why is this so challenging? The reasons are not surprising:
- Extensive outsourcing of manufacturing, packaging, labelling and distribution across often multiple supply chain partners, which results in more challenging collection of required data.
- Lack of integration and assembly of key IRT data covering site performance in patient enrollment as well as distribution supply chain performance. As IRT data resides in different toolsets per study, this data can often be misaligned with the internal clinical supply management system. The effort to standardise the information and its structure is challenging and significant.
- Lack of a proper or existing IT data infrastructure to support the development of the required data hub structure for the analysis activity.
- Lack of access to skills ranging from project management to technical data to data integration and data structure design. These are all key for developing the capabilities needed to leverage the data assets.
- Lack of investment (perhaps resulting from lack of a solid business case) in data analytics capabilities for clinical supplies.
- Lack of clinical supply leadership prioritisation for achieving business intelligence (when compared to the ongoing challenges of coordinating and executing the clinical supply chain for often hundreds of active studies).
- Lack of proper protection, governance and ownership for the handling of personalised data
As a result of these challenges, the resulting status quo for clinical supplies data analytics capabilities is limited, with most companies managing their clinical supply chains on a study-by-study basis and making major operational decisions based on experience, without the benefits of data-driven analysis to foresee or respond to the supply chain challenges.
For many, this reality means that basic understanding of current performance beyond the study level is highly limited. And any specific needed answers or insights require significant manual efforts to produce fully believable results.
There are bright spots; these are the leaders that have passion, belief, and determination to harness existing data for analysis leading to better planning and execution decisions for their management of clinical supply chains. These leaders can be characterized by the following statement:
They are in control of their data and know their performance.
To be in this position, these leaders have already developed their data assets and capabilities per the points below:
- Consolidated their data repository data model to include data from multiple sources to cover the end-to-end clinical supply chain
- Gained and leveraged their historical study performance data
- Assembled a data analytics toolset for analysis and distribution of information
- Developed an in-house staff for the ongoing pursuit of data intelligence
- Created a culture that fosters the understanding that this effort is a journey of learning and improvement
- Joined key external initiatives where latest technologies are being leveraged and look for outside capabilities and ideas as inspiration
- View business intelligence as a component of an overall digital transformation
The leaders realise that the path forward requires both risk and investment. But by gaining base capabilities for knowing their current performance and being able to explore next-level capabilities to assist in strategic decisions, they have reached the place where going backwards in their abilities or standing still is just not an option.
TOP USE CASES FOR CLINICAL SUPPLIES BUSINESS INTELLIGENCE
Business intelligence can be understood as a supportive capability for answering both tactical and strategic business questions. Answers to such questions lead to a progressing hierarchy of data analytics capabilities.
How are we doing? This is monitoring the current state and summary performance of the clinical supply chain on a near real-time basis. Once this fundamental question is addressed the concern becomes:
Where can we improve our decision-making or our processes? Here begins the need for insights which starts with the analysis of the data and the preliminary conclusions for operating more effectively. With insights in place for how decisions or actions can make a current impact, comes the next question:
How will these decisions or actions project into the future? This is the ability to forecast the impact of the decisions and how they will affect performance. Combined with the earlier two capabilities, this can set in motion continuous improvement, which then leads to:
What is the optimal way to operate? Here, business intelligence is supporting the longer-term strategic decisions of managing the clinical supply chain in ways that benefit overall drug development goals.
Below are several advanced approaches and capabilities that some clinical supplies organisations are actively pursuing or have in place:
- Study supply chain network optimisation: Leveraging prior performance results, trends, and projected costs and lead times/timeframes across network design options to impact the study supply chain design.
- Patient enrolment forecasting: Leveraging prior patient enrolment data per the therapeutic and country elements along with site performance data to model more accurately the projected patient enrollment for the study.
- Dashboards for current state and predictive results: Near real-time performance data at the study, programme and therapeutic area level to inform the health of the clinical supply chain.
- Cost analysis for historical and FY plan: Capturing of cost actuals and estimations to permit cost analysis of options in consideration.
- Third-party performance management: Visibility into performance factors versus plan or contract to enable improved selection and more targeted oversight practices.
THE ROAD TO ACHIEVING CLINICAL SUPPLY CHAIN BUSINESS INTELLIGENCE
As many in the industry have yet to launch an initiative towards data-driven business intelligence, here are some recommended steps to get underway.
- Assemble a small team to understand what is possible in your environment, and what the desired end state could be for business intelligence. Establishing the potential business value is also a good idea.
- Focus first on understanding what data is immediately available. Plot out how to acquire the data and begin to understand the required data model. Find out what existing IT assets and tool sets exist; among industry leaders in this area, just about no one starts from scratch.
- Identify the initial targeted use cases; these often address the first two questions ‘how am I doing?’ and ‘where can we improve our decision making process?’
- Develop the initial capabilities. This should be done in an agile manner with lots of end-user involvement and the expectation that iterations and lessons learned are part of the journey.
- Establish leading capabilities. Once initial dashboards and summary analytical data is in place as proof points, then leading capabilities can be pursued. Here we see leaders involving complex algorithms and artificial intelligence onto their data sets to optimize planning and execution parameters.
THE FUTURE: THE INTELLIGENT CLINICAL SUPPLY CHAIN
The longest-lasting impacts of the application of business intelligence in any business area are:
- The ability to create a new normal of data-centric management decision practices
- The momentum that verified data producing measurable intelligence can achieve in providing evidence forward into continual improvement efforts
With clinical research and development continuing to be the driver of future growth and market success, the clinical supply chain must keep pace and deliver value in an increasingly complex clinical trial world. We see a future where the clinical supply chain matures in its capabilities and management practices to operate closer to the capabilities of the commercial supply chain: more predictive, more efficient, better planned, and with more agility to achieve study objectives.
One major enabler for the near future will be increased usage of platform solutions and data standards to vastly improved integration across partners. We expect this interoperability to become a new norm. Leveraging, in near real-time, the end-to-end clinical supply chain data combined with a rich data set of historical data will be a necessary capability for managing the increased complexity and higher required performance standards of the future.
A FINAL WORD
This series of articles has covered four separate topics of clinical supplies management excellence. The topics were released in succession starting with a focus on the clinical supply organization and its agility, to the importance of end-to-end supply chain visibility, to then the key elements of clinical supply chain planning excellence, and then finally to this article covering the value of business intelligence for the management and key decision making needed in today’s complex world of clinical supply chain management.
We hope that the insights and ideas presented in this series are considered as opportunities to improve a most vital subsection of the industry.
Click here to read Pursuing Excellence in Clinical Supply Management – Topic 1: Workforce and Agility.
Click here to read Pursuing Excellence in Clinical Supply Management – Topic 2: End to End Visibility.
Click here to read Pursuing Excellence in Clinical Supply Management – Topic 3: Planning Excellence.
Stay up to date with the latest #Lifeattenthpin #LifeSciences #Pharma #MedDevices #Biotech #Digitalforlife #Thoughtleadership #Medical Technology #AnimalHealth news by following us on Instagram #LifeAtTenthpin Facebook Tenthpin and our Tenthpin LinkedIn corporate page.


