Pursuing Excellence in Clinical Supply Management – Topic 1: Workforce and Agility
Tenthpin is launching a short series of articles covering aspects of differentiation and excellence for clinical supplies management. These articles are collections of experiences and insights which have come from working with the industry on various initiatives over many years. At Tenthpin, we have enormous respect and often awe at the achievements and impact of clinical supply chain professionals across the globe, and we offer this content as a way of illuminating and perhaps enhancing their performance.

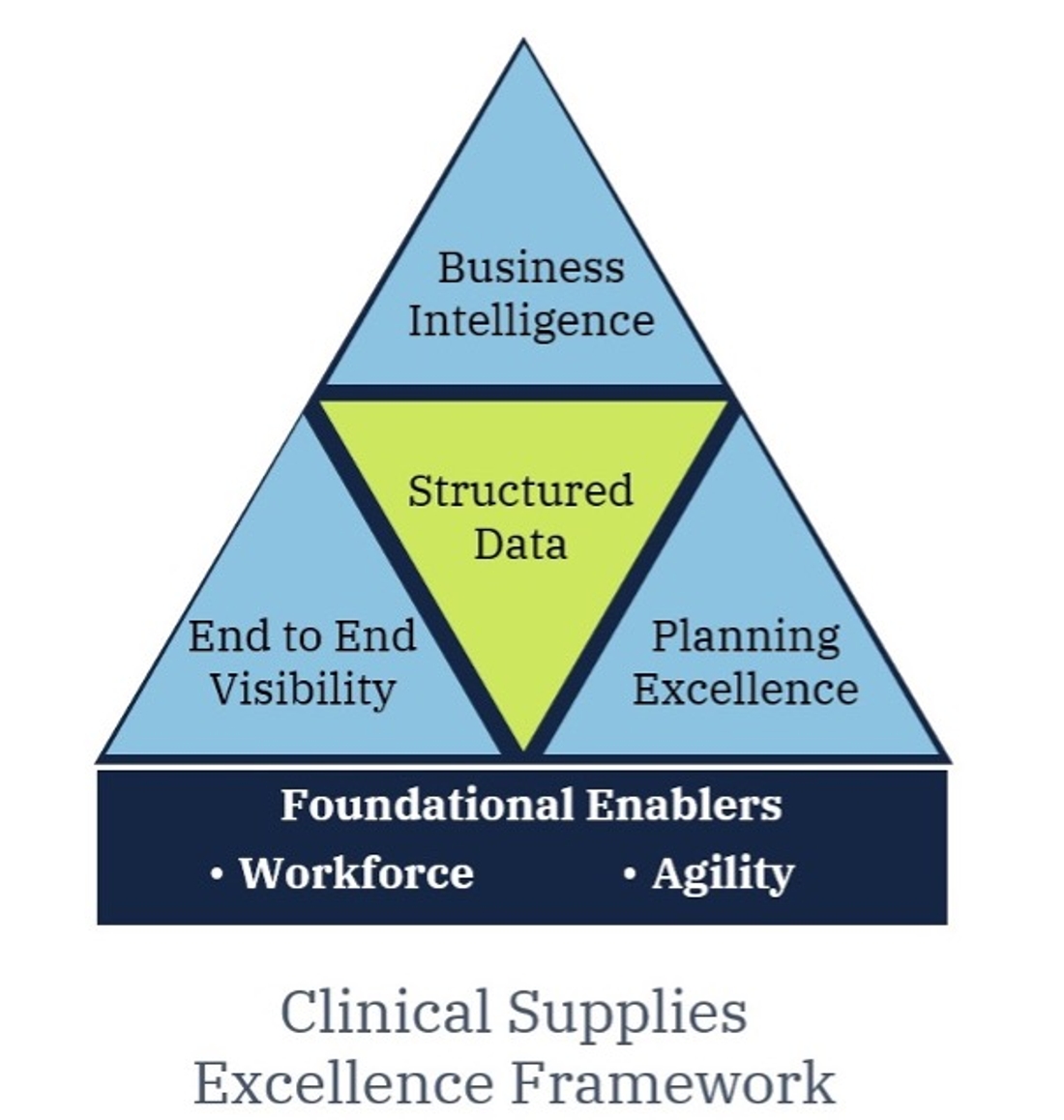
What follows is Article 1: Excellence in Workforce and Agility. Upcoming articles in this series will examine the more operational capabilities of excellence including end to end supply chain visibility, planning excellence, and business intelligence.
Introduction
Clinical research and development is the life blood of life sciences innovation. The design, execution, and oversight of the clinical trial supply chain requires a special breed of clinical trial and supply chain professional. The variety and complexity of this responsibility can often be stunning; these trials are each unique experiments conducted on volunteer patients driving toward the safety and efficacy of new therapies. These therapies represent the most advanced research, technology and insights into diseases of the human condition. And modern clinical study designs reflect the urgency to progress the novel therapies into approved state to treat patients; an urgency which is omnipresent across patient communities, regulatory agencies, and drug development professionals.
The role that clinical supply chain professionals play in these studies is crucial, demanding, and often overlooked. This is likely due to the high success rate – approaching 4- or 5-sigma levels – across pharma of achieving on-time and in-full clinical trial patient dosing. However, success in this most important metric is not to say that significant challenges do not exist. The clinical supplies area in pharma companies operates in different business models and organization structures, often using very different application platforms and having differing internal measures of success. This variety in operating models and measurements can make achieving comparative excellence in clinical supplies an elusive target.
Looking beyond the capabilities and technology of the modern clinical trial supply chain there are the foundational underpinnings of your clinical supply workforce and their ability to execute in an agile manner. These two are essential for delivering differentiated capabilities and measurable results toward clinical supply excellence.

Workforce excellence
Managing a clinical supply chain requires a unique set of skills, discipline, and grit to handle the inherent complexities and to adapt to ever-changing strategies and supply chain execution challenges. No factor is more important than your workforce to rise to these challenges and deliver excellence in clinical supplies management. First, let’s take a look at what many believe is the status quo for the clinical supply workforce and its work environment:
- Dedicated professionals whose loyalty and comradery within the clinical supply chain profession is very strong
- A cost-focused business environment where limited resource growth and increased outsourcing has led to aspects of change resistance or sheltering
- Sporadic investments into IT solutions and process capabilities
- Ever-increasing demand for more sophisticated clinical study execution; cold chain, titration schemes, combination therapies, adaptive trial designs, and more
In summary, the picture is one in which the workforce has been underinvested and underserved. In many organizations, an operational focus of ‘do more with less’ has continued throughout much of the past ten years – stressful indeed for your workforce.
As we are in a time of increasingly dynamic and rapid changes in drug development, what is the ‘new normal’ that your workforce is facing?
- Continued growth in clinical trials coupled with higher drug costs and the need for speed in drug development has turned the spotlight onto the clinical supplies function.
- Investments in improvement programs, often coordinated at the enterprise level for IT investments, or at the R&D level for capability improvements are emerging strongly. For these investments to move forward return on investment (ROI) remains the key determinant.
- There is a rapidly emerging talent shortage for clinical supplies professionals. This situation may cause greater separation in clinical supplies performance within the industry as in periods of combined rapid change and growth the axiom of ‘the best team wins’ applies. Overall, talent is getting more expensive, and increased turnover is eroding organizational performance since processes are often not documented or standardized within companies.
Let’s now discuss some areas where your workforce can be improved. Going back to the ‘best team wins’ analogy, we believe that you may find workforce investment and improvements have outsized returns for your capability and operational improvements – an upward spiral well worth the efforts. A few key questions:
Have you done workforce planning?
Establish your current capabilities and needs and now think ahead 3-5 years to map out your future resource and talent needs. Your initiatives and their corresponding investments can be a big component of the resource development roadmap to that future workforce state.
Have you taken a fresh look at your talent development programs?
There are operational skills aligned with roles, and there are also skills related to the emerging technology and new processes/ways of working. Not mapping and acknowledging these gaps and then taking actions to expand the skillsets of your workforce can lead to career uncertainty across your team. A key best practice is to ‘fast track’ the training process for new hires allowing them to contribute to the organization faster.
Is your workforce part of the journey of change and improvement?
Change resistance is much lower when the workforce is included in the plans and the execution of the changes – so long as the imperative for change and the change vision to be accomplished remain clinical supplies leadership responsibilities. Allowing the workforce to be part of the change is a matter of both trust and investment in them towards the future.
In our consulting work we often observe two situations when we host topic-based interactions across multiple companies: the presenter organizations and the learner/questioner organizations. This dichotomy is perhaps a leading indicator of where workforce knowledge and confidence reside. For the less confident, it can be remedied through greater internal resource enablement, more attendance at conferences, and encouragement by management to share experiences and challenges for discussion – becoming a ‘speak-up’ culture.
While this future may be unclear, debated, and driven outside of the clinical supplies function, the hybrid workplace is certainly one to be considering – for those roles where it can be sensible. One clear advantage is greater recruitment possibilities and as a downside potential increased turnover as employees have opportunities beyond their local area.
The good news regarding your clinical supply workforce is that employees have a strong personal value commitment to their chosen career. For these professionals, satisfaction and fulfilment primarily comes from achieving the goal of serving the patient and this is an enduring value.
Next, let’s look at the new imperative for your clinical supply organization and its workforce – agility.
Agility excellence
In today’s environment, agility is a highly used claim. As the opposite of agile is ‘stiff’ and ‘slow’ it is easy to see why individuals, organizations, supply chain partner organizations, and even software solutions are claimed to be agile. It’s helpful to take a step back and look at what ‘agile’ really means in terms of clinical supply management.
Across their normal daily work clinical trial supplies professionals are often called upon on to evaluate and react to:
- Changes in the clinical trial protocol or study parameters
- Changes in the patient enrolment forecast or in the actual enrolments
- Supply chain planning or execution challenges
Reactions to any of the above are a part of ‘normal’ business circumstance within clinical supplies. They are situational and often per an individual clinical trial; as such they are not really examples of organizational agility, but instead examples of business responsiveness.
Ok, now how about agility? If we look beyond the day to day to identify clinical supply business capabilities needed across clinical trials, or business functions, or even organizations we enter into something more strategic with higher business value. This is where an organization and its processes needs to be agile in order to handle situations such as:
- Scale up / scale down to meet R&D clinical trial demand fluctuations
- Prioritize or segment clinical trials to fast track the clinical supply process and increase speed per business objectives
- Pivot and adjust to broad supply chain challenges
- Strategically assess and act to provide operational flexibility to the clinical supply chain

With this positioning of agility into a more strategic sense, what are some of the key areas of excellence for attaining agility?
- Awareness and visibility to future state needs and challenges.
- This is needed for a more accurate picture and sense of timing for achieving the target agility(s). It is achieved through relationships and inter-organizational structures and governance.
- Leaders in clinical supplies are well interconnected to their counterparts in clinical operations and involved in key decisions and future plans.
- A strategic mindset across the clinical supplies leadership team to understand, plan and communicate how agility will be achieved.
- Be as specific as possible to paint a picture of the desired future state capabilities and their impact.
- Once established, the leaders in clinical supplies must embrace the change and be consistent in communicating the need to change.
- A path forward specific to the target agility(s) that has key stakeholder awareness and buy-in beyond the clinical supplies function.
- Often the agile requirement will initiate outside of clinical supplies, such as within new trial strategies or designs, the push for being sponsor of choice, or improved patient-centric capabilities.
- These drivers will require close alignment and coordination at the key stakeholder level for appropriate funding and governance as the costs and benefits are likely across organizations.
Summarizing the above, becoming agile requires an open recognition of challenges and future needs, the desire to continually improve, alignment with broader R&D and drug development goals, and enduring leadership to communicate and lead the change efforts.

Becoming more agile in your operations is an expression of confidence in your team’s abilities to meet some of the major challenges in today’s drug development.
Conclusion
Your clinical supply workforce and their capacity to be both responsive and agile are the foundation for positive and enduring improvements in clinical supply performance. These two factors span your operational strategy and your capacity to execute it and are thus essential to the operational excellence capabilities of end-to-end supply visibility, planning excellence, and business intelligence which we will explore in successive articles.
Stay up to date with the latest #Lifeattenthpin #LifeSciences #Pharma #MedDevices #Biotech #Digitalforlife #Thoughtleadership #Medical Technology #AnimalHealth news by following us on Instagram #LifeAtTenthpin Facebook Tenthpin and our Tenthpin LinkedIn corporate page.

