How to digitally transform as a Contract Development Manufacturing Organization
Contract Development Manufacturing or Contract Research & Manufacturing Services Organizations take-over more and more activities from “traditional” Life Sciences companies. CDMO services span from Drug Formulation Development, Clinical Trial Services, API production, drug product production to packaging operations.

Introduction
Recent studies predict a growth of approximately 7% CAGR resulting into a total revenue of around 160 billion $ of all global CDMO business in 2025 and therefore 2-3%-points above end-market. Growth is mainly driven by three drivers:
- Pharma companies put stronger focus on their core competencies (product development and distribution) and development of complex drugs incl. biologics
- Increasing focus on integrated end-to-end CDMO offering
- (Continuous) increase of regulatory requirements for the CDMO sector
As the overall CDMO business will significantly grow over the upcoming years CDMOs need to digitally transform to become agile, resilient and flexible enterprises to cope with dynamic client requirements. Even after a consolidation in last few years, the current CDMO market is still rather fragmented by multiple regional small-medium size players world-wide.
But this will change over the next 3-7 years. CDMOs will acquire other niche players to improve their service offering and reduce Supply Chain costs. But also Private Equity funds identified the CDMO market as highly interesting field to play. There will be massive and permanent consolidation and Merger & Acquisition activities.
(Source: PharmSource Trend Report, 2020)
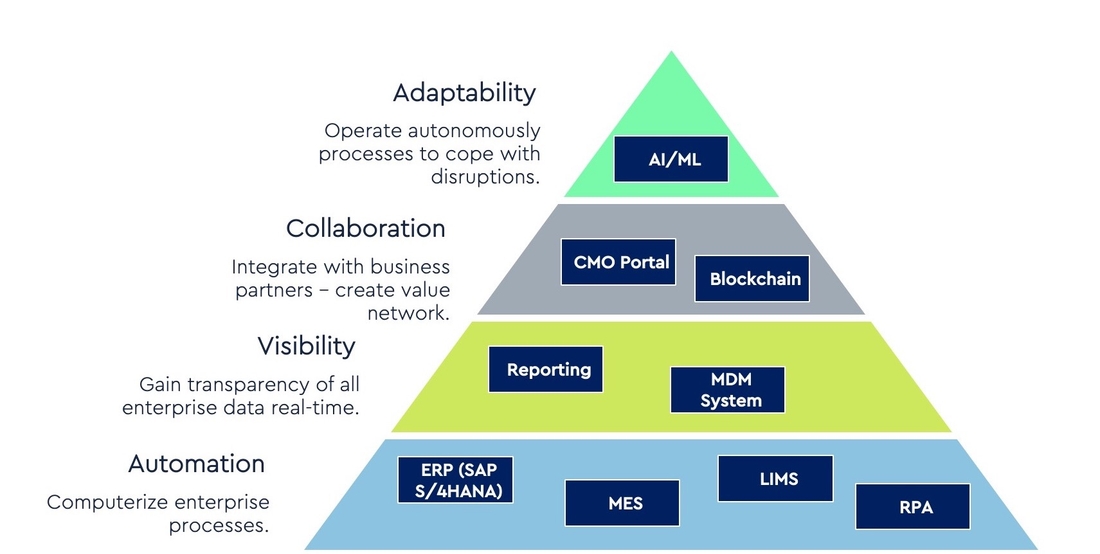
Digital Journey for CDMOs:
This ongoing consolidation and M&A activities in the CDMO market requires from CDMOs to be / become not only more service-oriented but especially:
- Adaptive
- Standardized
- Collaborative
- Transparent
- Automated
In order to become “fit-for-future” many CDMOs started to create their Digital Agenda to transform their organization for the upcoming years.
We recommend to build the foundation first by automating enterprise processes. An integrated ERP system like SAP S/4HANA enables to computerize all enterprise processes like Procurement of raw materials, production of drug substances and drug products, delivering the produced drugs to the pharmaceutical customers.
Introducing Tenthpin CDMO Model
The Tenthpin CDMO Model is a starter-package for all CRO and CDMO companies from pure Research & Development organizations, Clinical Trial enterprises, to full-fletched R&D -Manufacturing - Distribution organizations.

The Tenthpin CDMO Models contains three key elements:
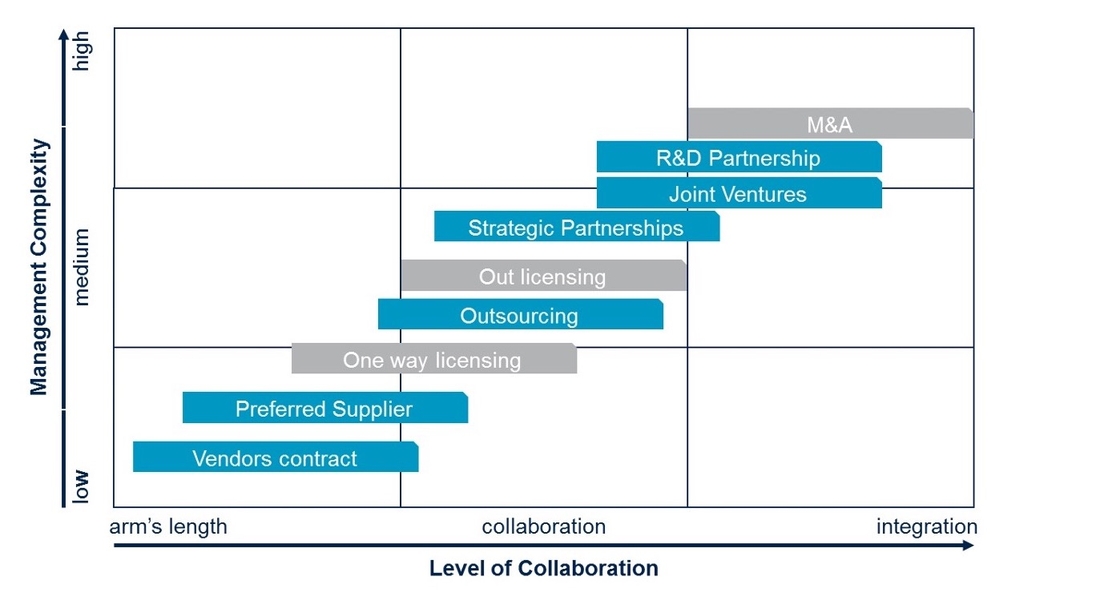
1. Client Interaction / Integration Models
There are multiple ways for CDMOs to interact with their clients. A key decision driver for setting up the right Interaction Model is the depth of collaboration/integration with the clients. Based on Tenthpin experience most clients and CDMOs share certain data like customer forecasts (from clients) or production capacities (from CDMOs).
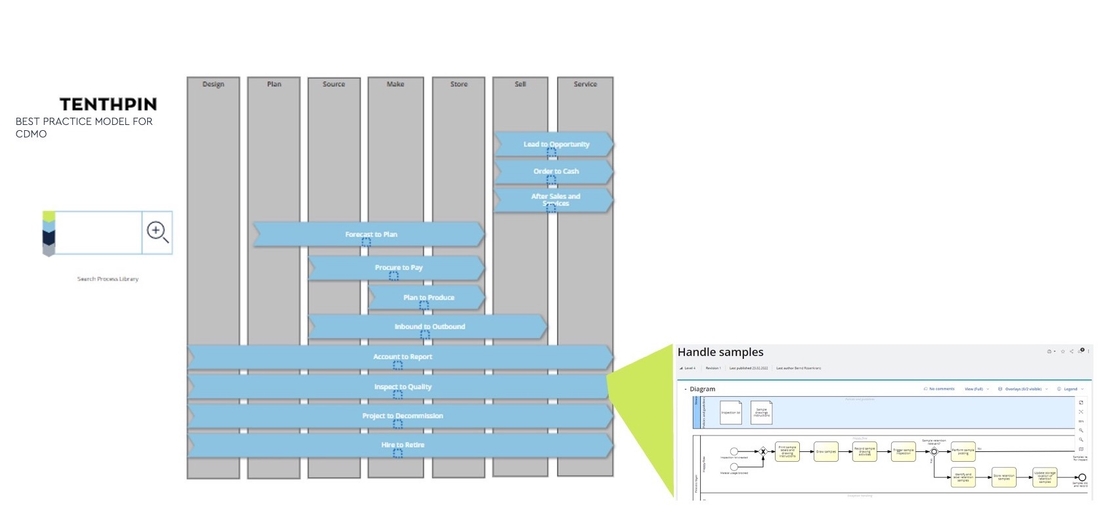
2. Enterprise CDMO Process Model
Tenthpin developed an Enterprise Process Model for CDMOs. This Process Model spans from level 0 to level 5 (activity level) covering all enterprise process areas: Project-Start-to-Finish, Purchase-to-Pay, Plan-to-Produce, Inspect-to-Release, Inspect-to-Repair, Order-to-Cash, Store-to-Ship, Finance-to-Report and Hire-to-Retire. In all those process areas typical processes for CDMOs are modeled.
3. SAP S/4HANA CDMO Model Enterprise
Based on the CDMO Best Practices Process Model (described in 2. above) Tenthpin has a pre-configured SAP S/4HANA system covering all typically required CDMO processes including master data like material master, Bill-of-Material (encompassing all manufacturing stages from Drug Substance, Drug Product to Fill and Pack), Recipes, Business Partners (Vendors, Customers).
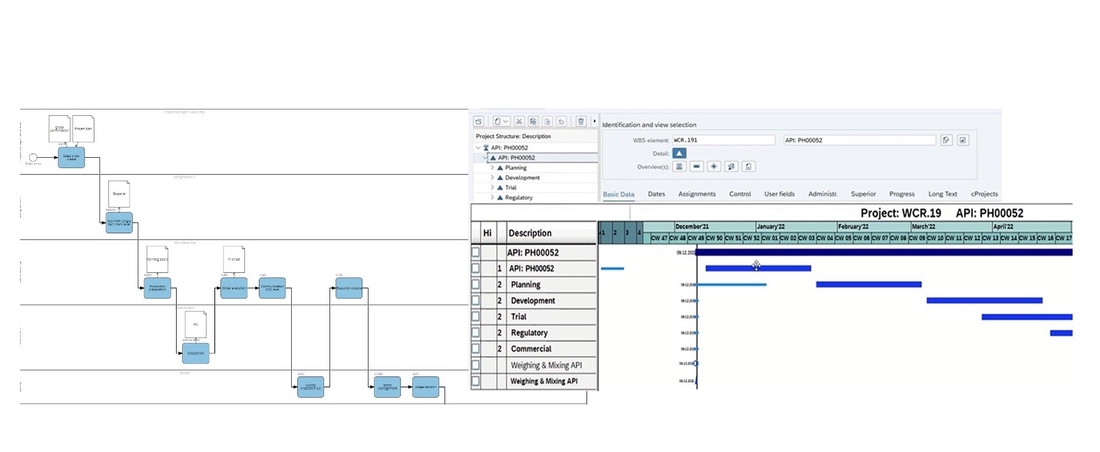
Discover Approach:
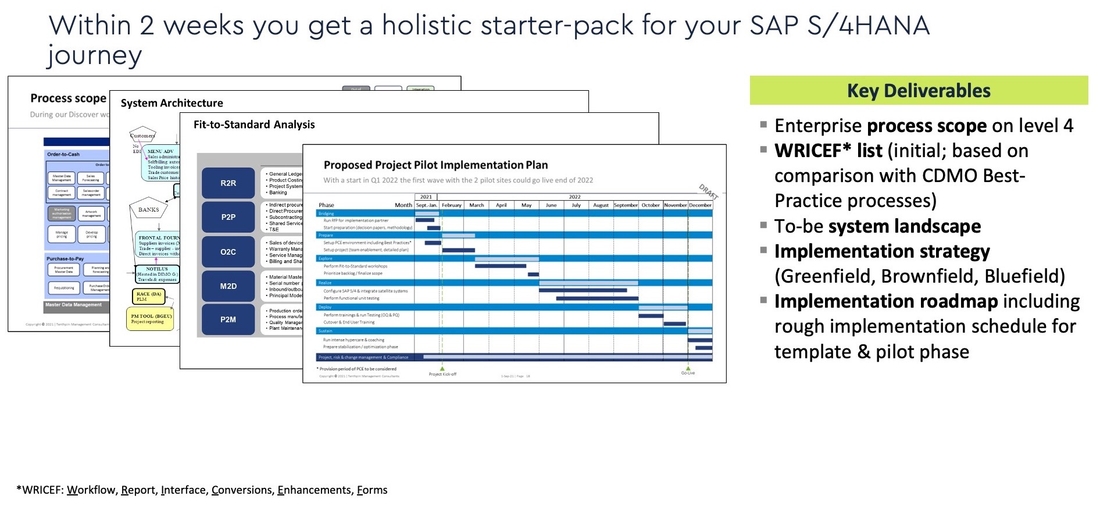
In order to get prepared for a SAP S/4HANA Transformation journey CDMOs can use Tenthpin’s Discover Approach to thoroughly prepare the implementation project. The main element is the Tenthpin Best Practice CDMO Enterprise Process Model which serves as a reference. As a result of this Discover phase a detailed scope, target application architecture, business case and implementation roadmap.
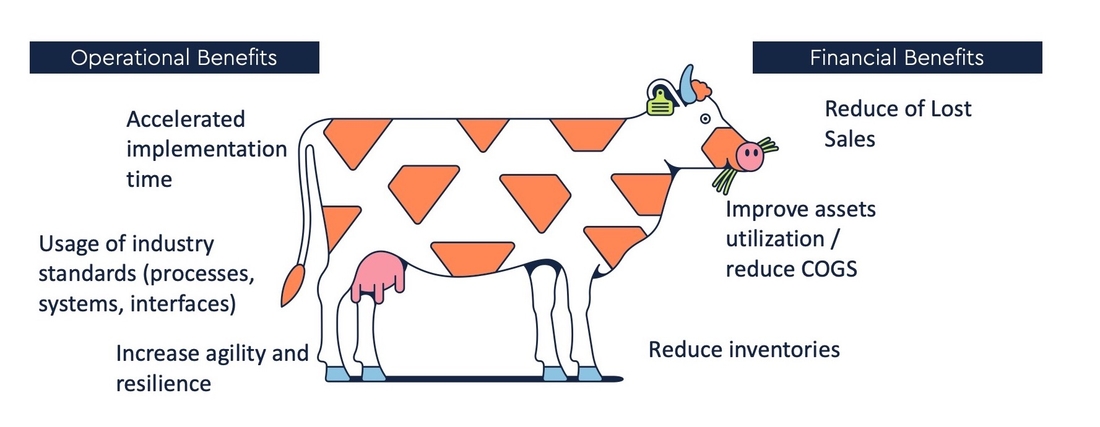
Benefits:
As a CDMO enterprise you will benefit largely from Tenthpin’s CDMO Model:
Conclusion:
The CDMOs landscape will significantly change over the upcoming 3-7 years because of consolidations, Merger and Acquisitions, introduction of new personalized drugs (Cell & Gene Therapies), new manufacturing technologies (e.g., “manufacturing-in-a-box”) etc.
These business changes require a reliable, flexible and integrated business model setup for CDMOs.
Therefore CDMO need to digitally transform their businesses. SAP S/4HANA is the aspired digital ERP core for many CDMOs.
Tenthpin has developed a holistic Digital Model for CDMOs containing an entire framework of Best Practice processes, pre-configured SAP S/4HANA system Template and Customer Interaction Models underlined by an entire set of templates (including CSV / GxP documents).
Many thanks go to Marco Gorgas for contributing to this article with all the CDMO market insights.
Marco Gorgas, former COO Aenova Group and Managing Director AGM LifeSciences GmbH: (1) Marco R. Gorgas | LinkedIn
Please reach out to our Life Sciences CDMO experts to find out more -> Process & Value Chain Consulting for Life Sciences | Tenthpin
Stay up to date with the latest #Lifeattenthpin #LifeSciences #Pharma #MedDevices #Biotech #Digitalforlife #Thoughtleadership #Medical Technology #AnimalHealth news by following us on Instagram #LifeAtTenthpin Facebook Tenthpin and our Tenthpin LinkedIn corporate page.

