Enabling real-world evidence use cases through data analytics
We have previously discussed the benefits and business cases of real-world data (RWD) and real-world evidence (RWE). However, the key question is how can value be added to business using this “new” data? RWE has been around for some time, but recent advances in analytics enabled new use cases. It can provide understanding on how patient characteristics and behaviors affect health outcomes (helping to predict the progression of a disease, a patient’s responses to therapy, or the risk of adverse events) while also increasing the efficiency of R&D investments and accelerating time to market. For any company considering implementing advanced RWE analytics, its success will depend on using the right framework and capabilities.

Introducing RWE
The introduction of advanced analytics in RWE has made real-world data a more powerful resource for pharma companies. Unlike traditional RWE methods —which use descriptive analysis to characterize patients, and established matching techniques to compare patient groups with similar characteristics—advanced RWE analytics uses predictive models, machine learning, and unsupervised algorithms to extract deeper insights from rich data sets. These techniques can help uncover insights into drug performance, run accurate scenarios with predictive models, and generate hypotheses for different drug development phases. More examples in the image below.
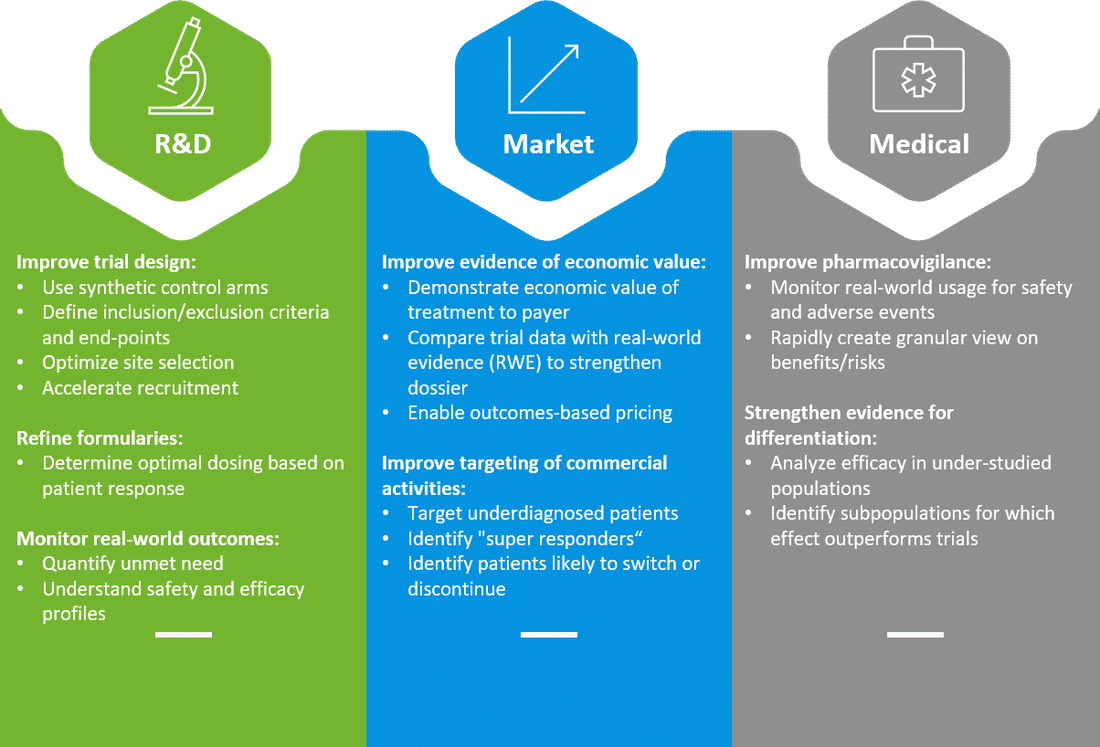
What are companies doing?
Leading companies are increasing their investment in building big data pipelines, reusable analytical assets and models, new and improved analytical platforms, and ecosystems across business areas (image below). This approach allows companies to integrate multiple datasets, use them to build analytical models, and then “industrialize” the models to apply them in a broad range of contexts. Over time, companies can embed the models in user-friendly digital tools for a variety of stakeholders, both internal (R&D, market access, medical science experts, and so on) and external (healthcare professionals, payers, patients, and others).
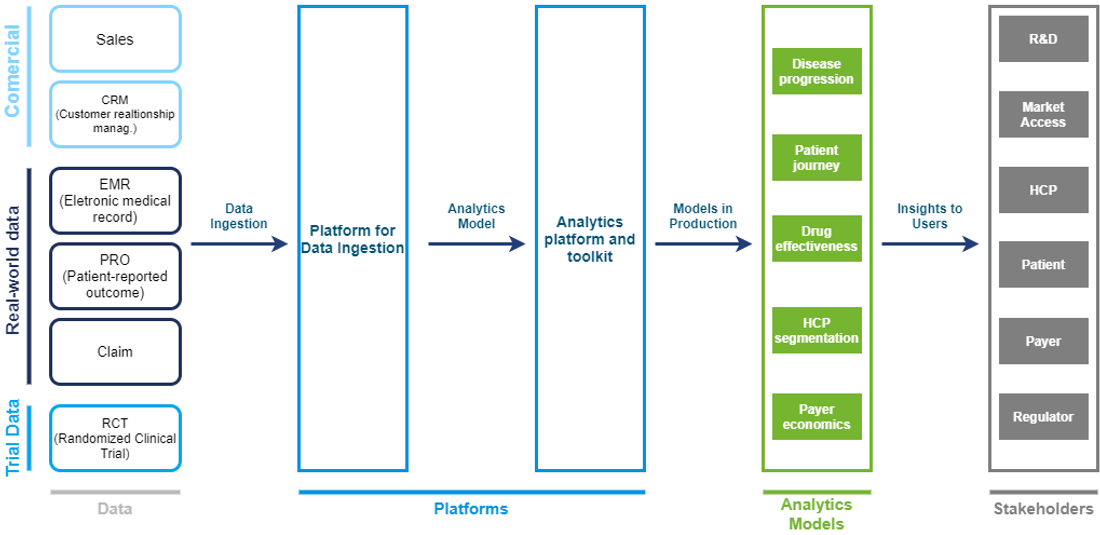
Our insights on RWE capabilities setup
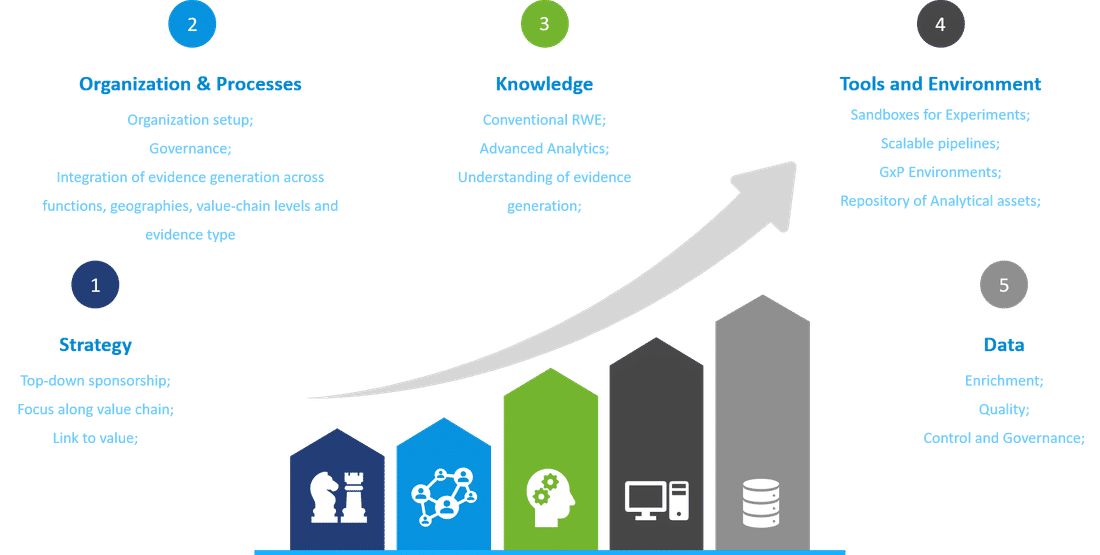
In combination with technology-enabled planning to integrate evidence generation across functions, these engines will transform RWE from a source of insights into a foundational pillar of corporate strategy and a key part of the value chain. Companies lacking this vision could struggle to compete with others that can base their decisions on richer insights generated at a fraction of the usual time and cost.
By taking full advantage of real-world evidence and advanced analytics, pharma companies can accelerate their transformation from product-focused to patient-focused organizations. Now it is time for the industry to set its sights on the next horizon of evidence-generation capabilities.
Please reach out to our Life Sciences Data & Analytics experts to find out more.
Stay up to date with the latest #Lifeattenthpin #LifeSciences #Pharma #MedDevices #Biotech #Digitalforlife #Thoughtleadership #Medical Technology #AnimalHealth news by following us on Instagram #LifeAtTenthpin Facebook Tenthpin and our Tenthpin LinkedIn corporate page.
